University Of Pittsburgh Scientists Discover Biomolecule That May Neutralize Coronavirus (Warning: Loud video starts automatically):
University of Pittsburgh scientists have isolated a biomolecule that “completely and specifically” neutralizes the virus that causes coronavirus.
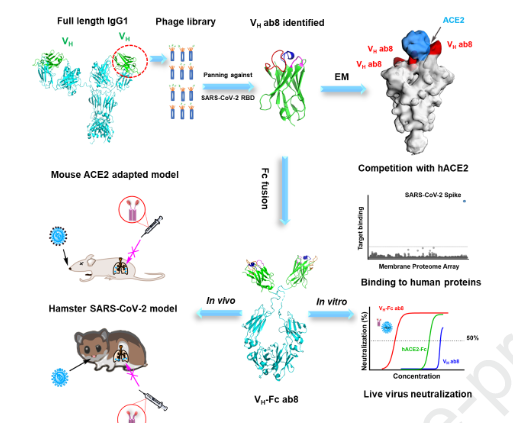
University of Pittsburgh School of Medicine researchers isolated the smallest biological molecule to date that neutralizes the SARS-CoV-2 virus, according to a report published Monday in the journal Cell. The antibody component is 10 times smaller than a full-sized antibody and has been used to create a drug known as Ab8 for use as a therapeutic and preventative against SARS-CoV-2, the report says.
The researchers reported that Ab8 is “highly effective” in preventing and treating SARS-CoV-2 infection in mice and hamsters.
I think — although the publication date is Sept 4, not Monday — the Cell article is the one titled, High potency of a bivalent human VH domain in SARS-CoV-2 animal models. (PDF) Its summary is a heavier lift:
We generated a phage-displayed human antibody VH domain library from which we identified a high-affinity VH binder ab8. Bivalent VH, VH-Fc ab8 bound with high avidity to membrane-associated S glycoprotein and to mutants found in patients. It potently neutralized mouse adapted SARS-CoV-2 in wild type mice at a dose as low as 2 mg/kg and exhibited high prophylactic and therapeutic efficacy in a hamster model of SARS-CoV-2 infection, possibly enhanced by its relatively small size. Electron microscopy combined with scanning mutagenesis identified ab8 interactions with all three S protomers and showed how ab8 neutralized the virus by directly interfering with ACE2 binding. VH-Fc ab8 did not aggregate and did not bind to 5300 human membrane-associated proteins. The potent neutralization activity of VH-Fc ab8 combined with good developability properties and cross-reactivity to SARS-CoV-2 mutants provide a strong rationale for its evaluation as a COVID-19 therapeutic.
Incidentally, on Sept. 14, Cell published Structurally resolved SARS-CoV-2 antibody shows high efficacy in severely infected hamsters and provides a potent cocktail pairing strategy, which also sounds promising:
Understanding how potent neutralizing antibodies (NAbs) inhibit SARS-CoV-2 is critical for effective therapeutic development. We previously described BD-368-2, a SARS-CoV-2 NAb with high potency; however, its neutralization mechanism is largely unknown. Here we report the 3.5-Å cryo-EM structure of BD-368-2/trimeric-spike complex, revealing that BD-368-2 fully blocks ACE2 recognition by occupying all three receptor-binding domains (RBDs) simultaneously, regardless of their “up” or “down” conformations. Also, BD-368-2 treats infected adult hamsters at low dosages and at various administering windows, in contrast to placebo hamsters that manifested severe interstitial pneumonia. Moreover, BD-368-2’s epitope completely avoids the common binding site of VH3-53/VH3-66 recurrent NAbs, evidenced by tripartite co-crystal structures with RBD. Pairing BD-368-2 with a potent recurrent NAb neutralizes SARS-CoV-2 pseudovirus at pM level and rescues mutation-induced neutralization escapes. Together, our results rationalized a new RBD epitope that leads to high neutralization potency and demonstrated BD-368-2’s therapeutic potential in treating COVID-19.
A PDF preprint is available online.

Will this discovery potentially leap frog over the vaccines that are in phase 3 trials?
I would imagine that to be very highly unlikely. It too would need the usual human trials to prove safety and effectiveness. And of course, not 100% of what works in animals works in people, not to mention the variation in side effects.